Introduction:
Every least square fit is subjected to
some degree of inavoidable arbitrariness which comes from different possible
choices of the least square sum and from unequally distributed existing
data. If the functions to fit are complicated, multiple possible solutions
are possible and some luck is necessary to find the best starting values
in order to come to the lowest possible least square sum. Therefore, fitting
of complicated functions like the Bender equation can be a longer task
especially if the functions to fit are not analytical but numerical iterated
functions. If the starting values are not appropriate the iteration can
lead to values where the algorithm breaks down.
That are the main problems one encounters,
we are interested now here only in the results.
Method:
The experimental phase equilibrium data
used to fit the Bender equation are given by [21].
As least square sum we summed up the relative
errors (Pex - Ptheor)/Pex and (xgex
- xgtheor)/xgtheor squared
of pressure and gaseous molar ratio of the dew line of the phase diagram.
Furthermore, in the end phase of a least square run, we added residuals
which contained "reconstructed theoretical" xfl and vfl
values and which were compared to the "experimental" theoretical value.
This "dirty" practical reconstruction
method is done as follows:
We define the "experimental" fugacities
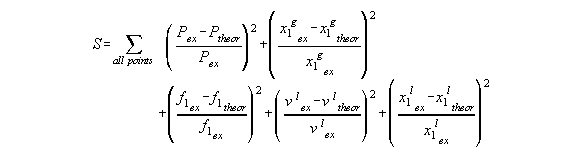
Results:
The fit constant of the most successful
run showed the most less deviation from the trivial value 1 for all fit
constants.
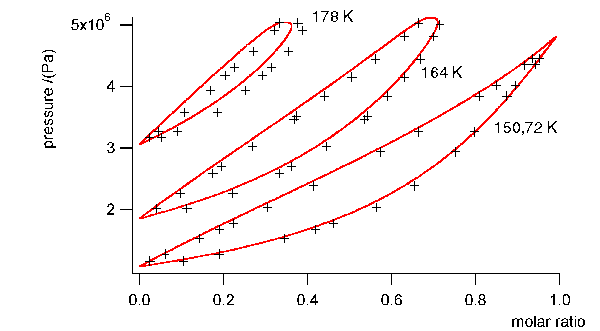
We used all points of the experimental
phase equilibrium points at high pressure and 178 K. In the end phase of
the iteration we included the reconstructed values in the least square
sum, but had to drop the experimental values at the two highest pressures
at 178 K, because then the algorithm would break down. Nevermind, afterwards
the iteration converged until the critical point.
In Fig. 1 the measured values are shown
compared to the calculated optimized solution from the Bender equation.
Table 1 gives the numerical values exactly including all of the relevant
material data.
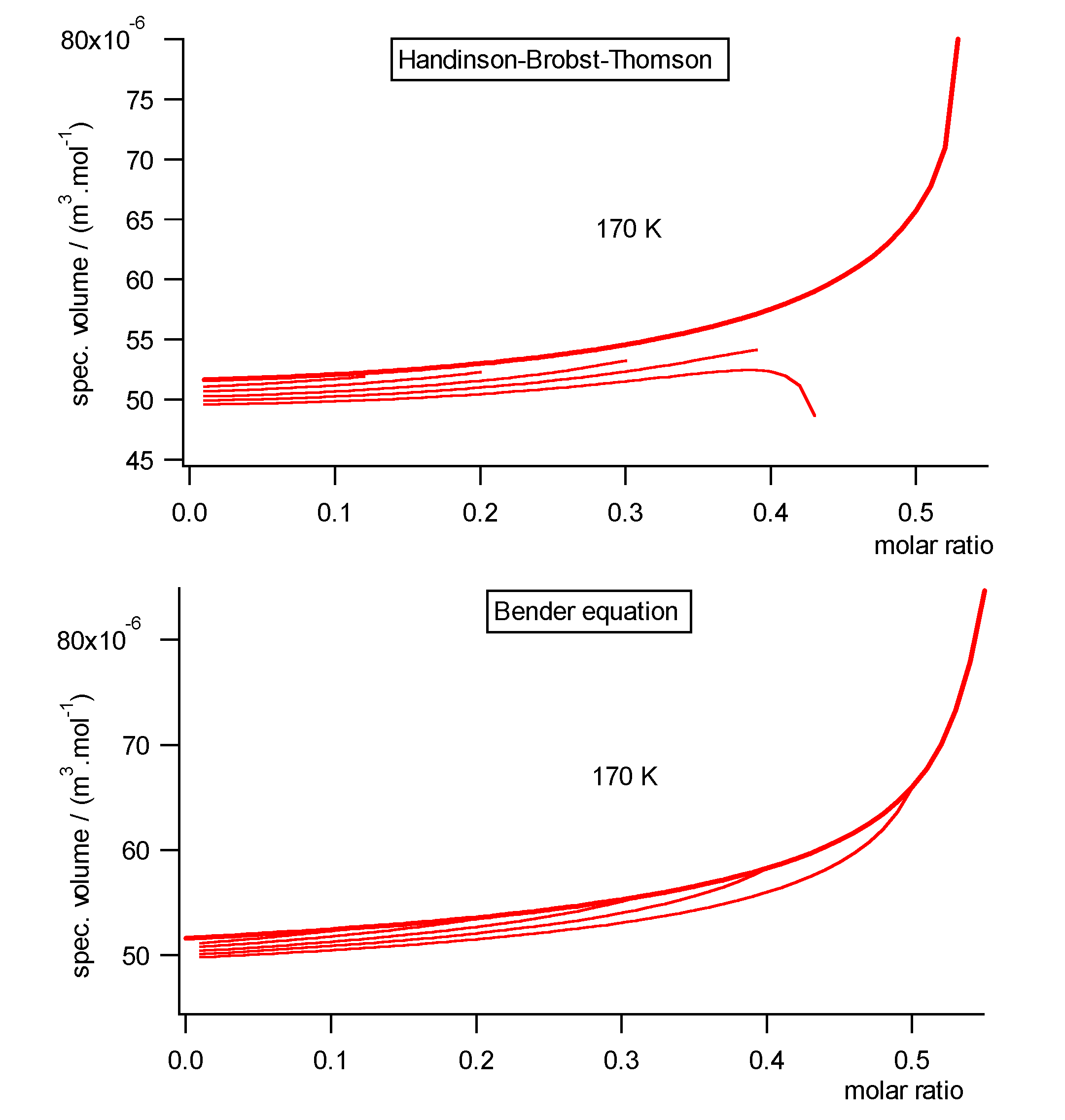
Fig.2 shows the liquid volumes at 170
K calculated with a standard Handinson-Brobst-Thomson (HBT) estimation
method [17] compared to the Bender equation. The estimation relation
breaks down in the neighborhood of the critical point contrary to the Bender
equation.
Discussion:
For our purposes the accuracy is sufficient.
Probably it so good that other values as enthalpies entropies and so on
can be taken as reasonable estimation values from the equation. It is clear
that the accuracy of the fit is higher at lower emperature because there
exist more data.
We remember that our equation is one solution
to describe the mixture. It is not the only or the best solution, because
if more and more exact data exist the mathematical modeling can to be refined
too.
Table 1:
Relevant Data of the Least-Square Iteration
_______________________________________________________________________
System: Argon -Methan
Material-file: a:ar_ch4.mix range of experimental
data: 150 - 180 K
Date: 12-07-1999 Time: 20:00:28
Material data:
Argon
Methane
mol. weight
39.948
16.043
crit. pressure
4865300
4598800
crit. volume
7.452985075e-5 9.9030865D-05
crit. temperature
150.69
190.56
Omega
-.00234
.0086
Stiel factor
.004493
.00539
ideal spec. heat polynom acc. to [17]
CPA
20.80
19.25
CPB
0
.05213
CPC
0
.05213
CPD
0
-1.132D-8
CPE
0
0
reference enthalpy
0
0
reference entropy
154.729
186.3384
HBT - parameter
crit. temperature
150.8
190.58
Omega
-.0092
.0074
crit. volume
7.5452985075D-05 9.930865D-5
ZR
.2922
.2892
reference state at
pressure
101325
temperature
278.2
Remarks to the least-square-iteration
Calculated least-square fit parameter of the mixture:
kij= .9977865068023702 Chiij=1.033181352446963
EtaM=2.546215505163194
Accuracy: single points
1.00D-07 Least-Squares-Iteration 1.00D-05
experimental values
No. T/K Xi/% P/bar
Xg/%
____________________________________________________________________________
1 150.8 2.5 1.174D+01
10.5
2 150.8 6.2 1.294D+01
19.0
3 150.8 14.3 1.553D+01
34.6
4 150.8 19.1 1.696D+01
41.8
5 150.8 22.3 1.798D+01
46.2
6 150.8 30.4 2.061D+01
56.3
7 150.8 41.4 2.417D+01
65.4
8 150.8 57.4 2.977D+01
75.2
9 150.8 66.5 3.305D+01
79.7
10 150.8 81.0 3.894D+01
87.4
11 150.8 85.0 4.068D+01
89.5
12 150.8 92.0 4.416D+01
94.2
13 150.8 93.5 4.512D+01
95.2
14 164.0 4.1 2.043D+01
11.3
15 164.0 9.8 2.288D+01
22.2
16 164.0 17.5 2.618D+01
33.3
17 164.0 19.6 2.736D+01
36.2
18 164.0 26.8 3.069D+01
44.5
19 164.0 36.8 3.514D+01
53.5
20 164.0 37.4 3.564D+01
54.2
21 164.0 44.1 3.897D+01
59.0
22 164.0 50.5 4.209D+01
63.1
23 164.0 56.2 4.505D+01
66.8
24 164.0 63.0 4.876D+01
70.0
25 164.0 66.5 5.099D+01
69.6
26 178.0 2.4 3.210D+01
5.3
27 178.0 4.6 3.309D+01
9.0
28 178.0 10.9 3.648D+01
18.5
29 178.0 16.5 3.985D+01
25.3
30 178.0 20.4 4.229D+01
29.2
31 178.0 22.7 4.366D+01
31.4
32 178.0 26.8 4.629D+01
35.5
33 178.0 32.2 4.976D+01
38.7
34 178.0 33.3 4.995D+01
37.6
errors between experiment and theory
No. T/K
Xi/% P/bar dP/% Xg/%
dXg/% Vfl
Vg
____________________________________________________________________________
1 150.8 2.5
1.165D+01 0.8 8.6 18.5
4.520D-05 8.834D-04
2 150.8
6.2 1.298D+01 -0.3 18.9
0.4 4.521D-05 7.846D-04
3 150.8 14.3
1.579D+01 -1.6 35.4 -2.3
4.522D-05 6.298D-04
4 150.8 19.1
1.740D+01 -2.6 42.6 -2.0
4.523D-05 5.630D-04
5 150.8 22.3
1.845D+01 -2.7 46.8 -1.2
4.524D-05 5.253D-04
6 150.8 30.4
2.110D+01 -2.4 55.5 1.3
4.529D-05 4.472D-04
7 150.8 41.4
2.468D+01 -2.1 64.9 0.8
4.542D-05 3.672D-04
8 150.8 57.4
3.008D+01 -1.1 75.6 -0.6
4.583D-05 2.811D-04
9 150.8 66.5
3.337D+01 -1.0 80.9 -1.5
4.629D-05 2.413D-04
10 150.8 81.0
3.915D+01 -0.5 88.8 -1.6
4.784D-05 1.846D-04
11 150.8 85.0
4.091D+01 -0.6 91.0 -1.6
4.865D-05 1.693D-04
12 150.8 92.0
4.424D+01 -0.2 94.8 -0.7
5.115D-05 1.410D-04
13 150.8 93.5
4.501D+01 0.2 95.7 -0.5
5.206D-05 1.341D-04
14 164.0 4.1
2.061D+01 -0.9 10.5 6.8
4.935D-05 4.850D-04
15 164.0 9.8
2.341D+01 -2.3 22.1 0.5
4.961D-05 4.171D-04
16 164.0 17.5
2.710D+01 -3.5 34.0 -2.0
5.004D-05 3.481D-04
17 164.0 19.6
2.809D+01 -2.7 36.7 -1.3
5.017D-05 3.324D-04
18 164.0 26.8
3.147D+01 -2.5 44.8 -0.7
5.071D-05 2.857D-04
19 164.0 36.8
3.617D+01 -2.9 53.9 -0.7
5.176D-05 2.338D-04
20 164.0 37.4
3.645D+01 -2.3 54.4 -0.3
5.184D-05 2.310D-04
21 164.0 44.1
3.965D+01 -1.8 59.4 -0.7
5.288D-05 2.017D-04
22 164.0 50.5
4.277D+01 -1.6 63.7 -0.9
5.430D-05 1.759D-04
23 164.0 56.2
4.562D+01 -1.3 67.0 -0.4
5.624D-05 1.535D-04
24 164.0 63.0
4.902D+01 -0.5 70.3 -0.4
6.069D-05 1.246D-04
25 164.0 66.5
5.060D+01 0.8 71.0 -2.0
6.618D-05 1.052D-04
26 178.0 2.4
3.219D+01 -0.3 4.8 9.6
5.645D-05 2.825D-04
27 178.0 4.6
3.363D+01 -1.6 8.8 2.5
5.686D-05 2.663D-04
28 178.0 10.9
3.767D+01 -3.3 18.4 0.4
5.826D-05 2.258D-04
29 178.0 16.5
4.119D+01 -3.4 25.3 0.1
5.997D-05 1.951D-04
30 178.0 20.4
4.358D+01 -3.1 29.2 -0.1
6.158D-05 1.755D-04
31 178.0 22.7
4.497D+01 -3.0 31.3 0.3
6.281D-05 1.642D-04
32 178.0 26.8
4.738D+01 -2.4 34.3 3.3
6.588D-05 1.438D-04
33 178.0 32.2
5.006D+01 -0.6 36.2 6.5
7.532D-05 1.113D-04
34 178.0 33.3
5.038D+01 -0.9 35.8 4.9
8.004D-05 1.019D-04
Captions: