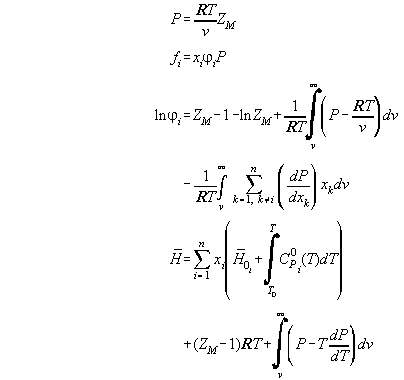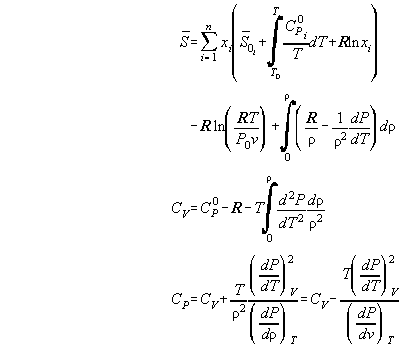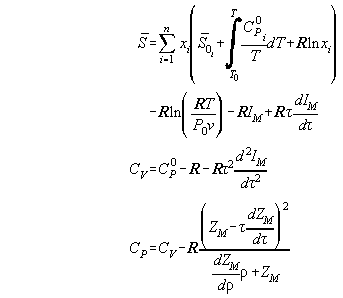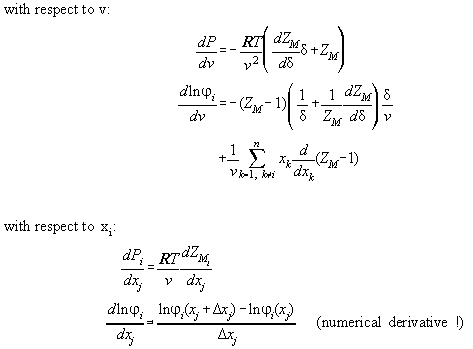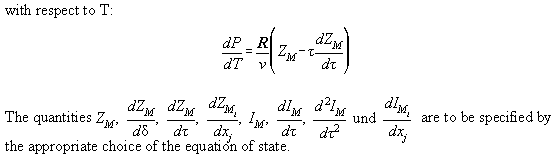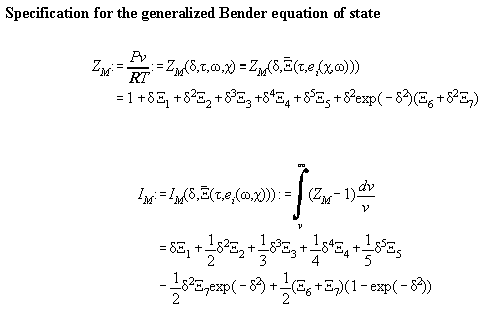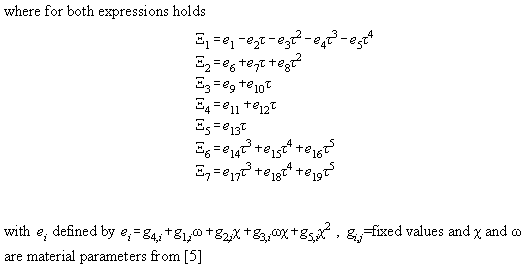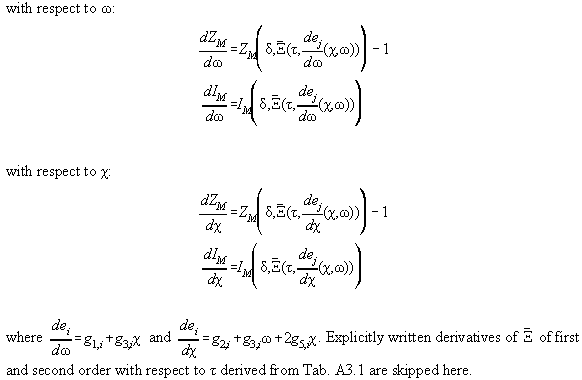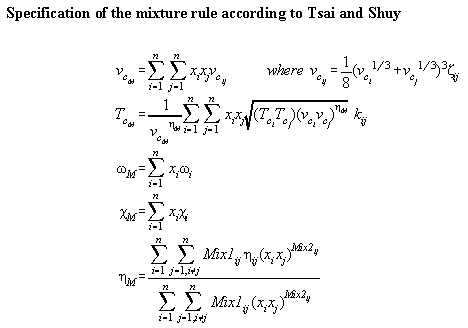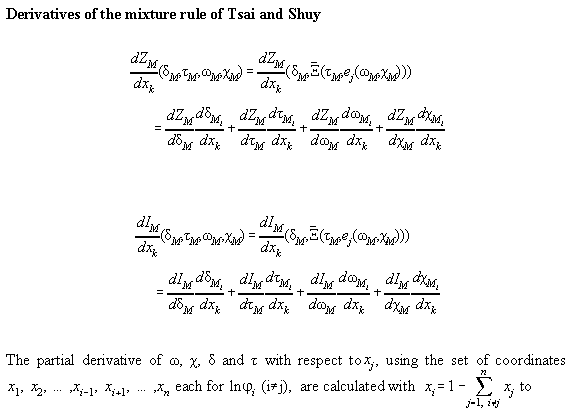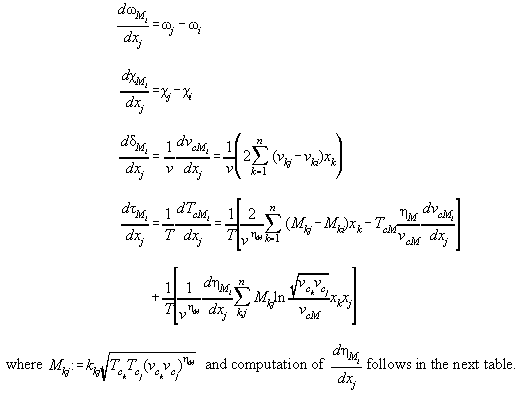Appendix 5: Calculation of the thermodynamic
state of a real fluid with a Bender equation of state
released 7.12.99, updated 9.12.99
Nomenclature:
latin symbols:
CP0(T)
ideal heat capacity (material parameter)
CV
specific heat capacity at constant volume
CP
specific heat capacity at constant pressure
ei
calculated constants of Bender equation of state
f
total fugacity
fi
partial fugacity of a component
g1,j
fixed constants of Bender equation of state
G
free enthalpy
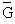 molar specific free enthalpy
molar specific free enthalpy
 molar specific enthalpy
molar specific enthalpy
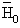 reference enthalpy at reference temperature T0 and reference
pressure P0
reference enthalpy at reference temperature T0 and reference
pressure P0
IM
defined as 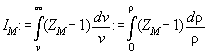
kij
fit parameter for mixture rule
Mix1
fit parameter for mixture rule for ternary and higher component mixture
Mix2
fit parameter for mixture rule for ternary and higher component mixture
P0
reference pressure
P
pressure
R
Avogadro constant
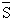 molar specific entropy
molar specific entropy
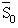 reference entropy at reference temperature T0 and reference
pressure P0
reference entropy at reference temperature T0 and reference
pressure P0
T
temperature
T0
reference temperature
Tc
critical temperature
v
specific molar volume
vc
critical specific molar volume
V
volume
W
work
w
specific work
x
molar ratio
ZM
real gas factor defined as Pv/RT
greek symbols:

indices:
upper indices:
f liquid (fluessig), g gaseous
lower indices:
i,j,k,l as component index or summation index written explicitly as number,
for example: x1 for component 1, M for mixture
numbers in brackets: for example v(1), x(2) means at point 2
one marked number: superheated state, i.e. v(3´) or x1(2´)
double marked number: two phase state, i.e. x(2") or P(3")
How to read the formula collection
Table A1 summarizes all standard formulas from literature describing
the differents aspects of a thermodynamic state. They can be found in [A1,A2,A3,A4].
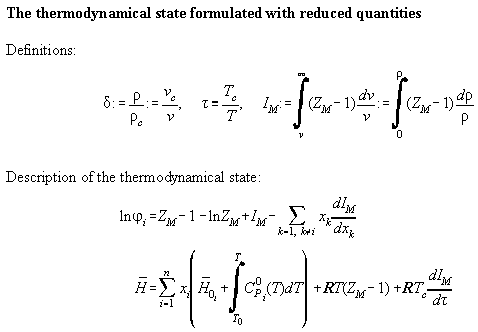
In Table A2.1 the set from Table A1 is written dependent from the reduced
quantities of volume 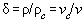 and temperature
and temperature 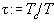
To make calculation more easy it is convenient to write the formalas
down in terms of ZM and IM defined as
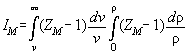 .
.
Derivations of Table A2.1 are found in Table A2.2. They are necessary
to calculate the Jacobimatrix of the Newton Raphson iteration. All formulas
of the thermodynamic state are analytically calculated. Only the the derivations
of ln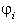 with
respect to xi are calculated numerically.
with
respect to xi are calculated numerically.
In Table A3.1 the Bender equation is specified, Table A3.2 contains
derivations therefrom necessary for the calculation of the Jacobimatrix.
Table A4.1 specifies the mixture rules of Tsai and Shuy[A5]. Table
A4.2 and A4.3 gives derivations therefrom.
For easy calculation of ln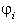 it is helpful to consider it to be dependent from (x1, x2,
xi-1,xi+1,....xn)
it is helpful to consider it to be dependent from (x1, x2,
xi-1,xi+1,....xn)
The calculation is presented here top - down, i.e. from general to
special. Programming has to be done in the reverse direction
Tab.A1:
| The thermodynamic state in standard notations
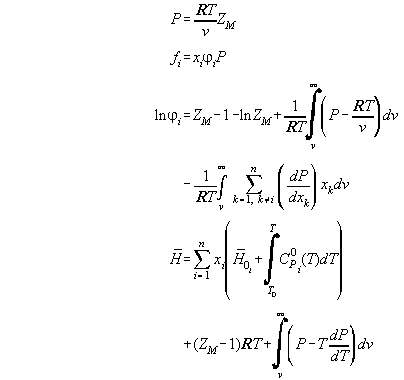 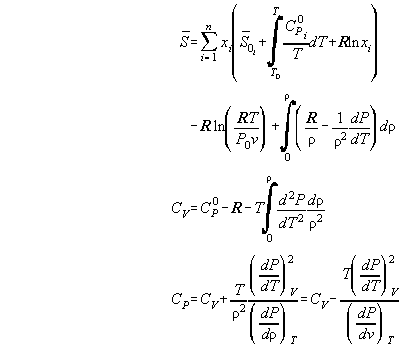 |
Table A2.1:
Table A2.2:
| Derivatives in reduced quantities
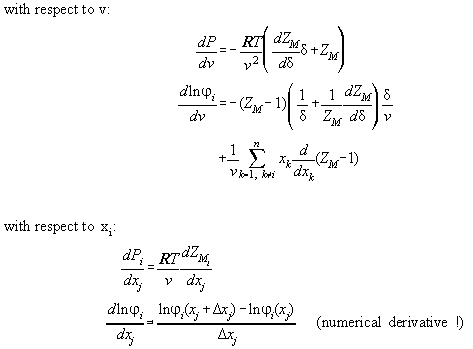
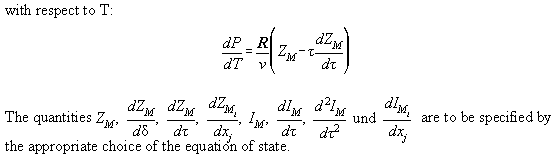
|
Table A3.1
Table A3.2:
Table A4.1:
Table A4.2:
Table A4.3:
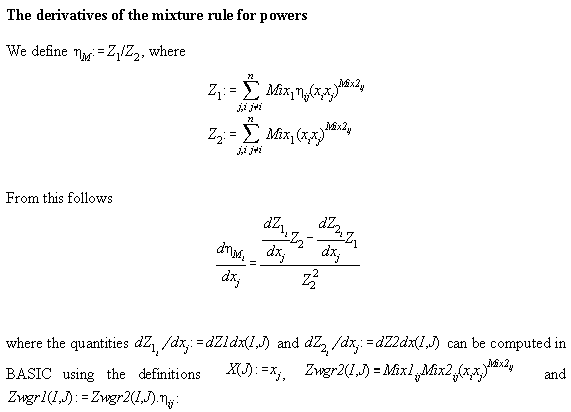
FOR I=1 TO N
FOR J=1 TO N
dZ1dx(I,J)=0 : dZ2dx(I,J)=0
NEXT J
NEXT I
FOR I=1 TO N
FOR J=I+1 TO N
FOR K=1 TO N
FOR L=K+1 TO N
IF I=K OR I=L THEN
dZ1dx(I,J)=dZ1dx(I,J)-Zwgr1(K,L)/X(I)
dZ2dx(I,J)=dZ2dx(I,J)-Zwgr2(K,L)/X(I)
ENDIF
IF J=L OR J=K THEN
dZ1dx(I,J)=dZ1dx(I,J)+Zwgr1(K,L)/X(J)
dZ2dx(I,J)=dZ2dx(I,J)+Zwgr2(K,L)/X(J)
ENDIF
NEXT L
NEXT K
dZ1dx(J,I)=-dZ1dx(I,J) : dZ2dx(J,I)=-dZ2dx(I,J)
NEXT J
NEXT I
|
Bibliography:
[A1] Bender E., Die Berechnung von Phasengleichgewichten mit der thermischen
Zustandsgleichung - dargestellt an den reinen Fluiden Argon, Stickstoff,
Sauerstoff und ihren Gemischen , Habilitationsschrift, Bochum 1971
[A2] Platzer B., Die Generalisierung der Zustandsgleichung von Bender
zur Berchnung von Stoffeigenschaften polarer und unpolarer Fluide und derer
Gemische, Dissertation, Fachbereich Maschinenbau, Uni Kaiserslautern 1990
[A3] Stephan K., Mayinger F. Thermodynamik, Springer Verlag, New York
1988
[A4] Polt A., Zur Beschreibung von reinen Fluiden mit BWR-Gleichungen,
Fachbereich Maschinenbau, Uni Kaiserslautern 1986/87
[A5] Tsai F., Shuy J.J., Prediction of high pressure vapor-liquid equilibria
by the corresponding-states principle, J. Chin.Chem.Engn. 16 (1985) 157
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> back
to the main document
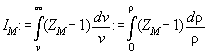

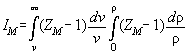 .
.